86-551-67709456 (Working time)
86-13965027700 (Nonworking time)
The European standard en13795 for disposable surgical gowns, sterile surgical drapes and clean air suits has released a new revision this year. The standard is divided into two parts. The first part discusses the requirements and test methods of surgical gowns and surgical drapes, and the second part discusses the clean air suit.

Performance requirements have basically not changed, but now we need statistical representation of data, rather than just providing all test data points. For a long time, people have questioned whether to use individual results or the average of all results to approve products. With the introduction of the "result processing" section of the standard annex, manufacturers no longer need to guess how to determine compliance. The test statistics include the consideration of the off topic results. Now, it is possible for the product to meet the requirements of the standard, which may have previously failed the test when checking individual results. The introduction of upper and lower quartile test statistics provides an easy to follow guideline for determining compliance. The new treatment of this result applies to all test methods except for the evaluation of the permeability of wet microorganisms (ISO 22610:2006).
For tests with maximum performance requirements, such as dry microbial penetration, microbial cleanliness, and particulate release, the upper quartile statistic, the upper middle half of the data set, is used. A test set whose upper quartile results meet performance requirements is considered compliant, regardless of whether all individual results meet the criteria.
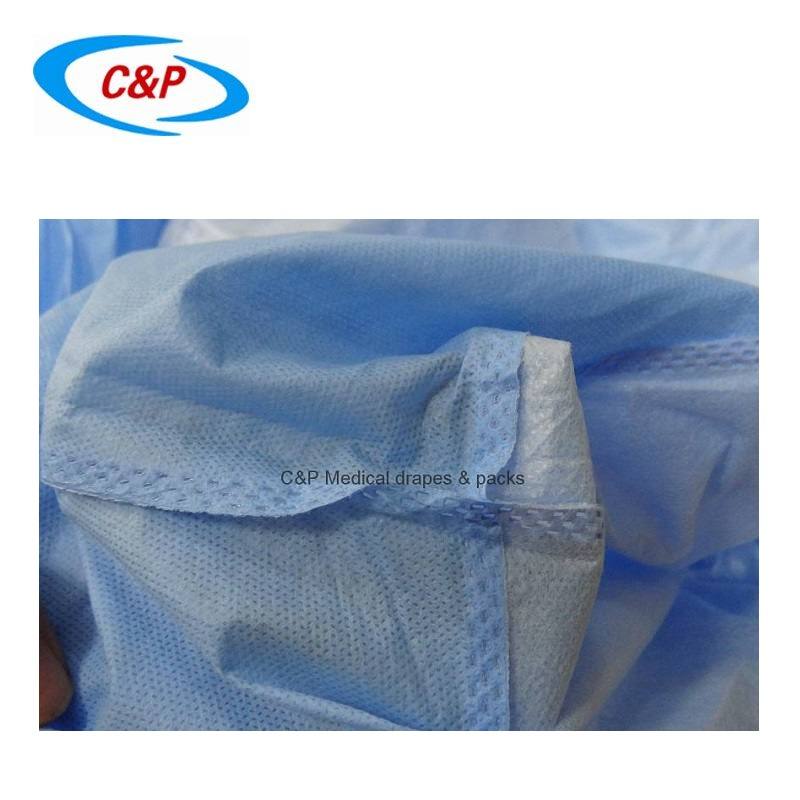
There is another change that could potentially impact manufacturers sampling plans for testing. Annex A indicates, “Testing shall include potential weak spots.” This statement was also in the previous revision but this new revision adds a note, which states, “In particular, all types of joints in critical areas, such as seams in sleeves of ultrasonic surgical gowns, are regarded as potential weak spots.” This new note makes it clear that manufacturers should test any seams or points of attachment within the critical area of the gown or drape as defined by the manufacturer. AAMI PB70, Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities, is one US analog of EN13795. This document has long included requirements to test potential weak spots such as seams and tie attachments, and gives specific requirements to the number to test for each area. The requirements for compliance with PB70 contain a full set of testing on each potential weak area in addition to testing the base material alone. While EN13795 does not define specific requirements about how testing of potential weak spots should be approached, the requirements in PB70 can be used to give guidance. Joint and seam areas in critical areas of products should be considered weak spots, and should be subjected to testing to ensure full protection for health care workers and their patients.

The changes to this requirement make the document easier to follow, and introduce a simplified approach to determining compliance with the performance requirements. Clearer instructions about how to treat the testing results will lead to fewer questions about compliance. Instructions for manufacturers and processors aid in their determination of an appropriate sampling plan.
Get in touch
Scan to wechat:
